Primary Versus Mixed Conditions
This is guaranteed to keep coming up on every exam for the rest of your
life, so you might as well learn it.
This page provides two ways to consider acid-base disturbances:
Method I. A rigorous method
which involves calculation of the expected compensations.
Method II. A quick and dirty method
to tell from a blood gas if a respiratory condition is simple or compensated.
Method III. Look on a nomogram.
Also see the discussion of how to interpret the base
deficit on a blood gas.
Method I. Expected compensations
| Condition |
HCO3- |
pCO2 |
| Metabolic Acidosis |
Lower |
Lower |
| Respiratory Acidosis |
Higher if chronic |
Higher |
| Metabolic Alkalosis |
Higher |
Higher |
| Respiratory Acidosis |
Lower if chronic |
Lower |
Adapted from Peds Nephrol 42:1365-1395
In acid-base disorders, there are expected compensatory mechanisms.
For instance, when bicarbonate is lost the primary process is metabolic alkalosis
and the normal response is compensatory respiratory acidosis (retention
of CO2). If the change in pCO2 or HCO3-
is equivalent to the expected compensatory response, the disorder is "simple".
However, if the compensation is outside the normal range, it is a mixed
disorder; that is, two primary processes are taking place simultaneously.
The expected compensation is for each condition is defined below:
Metabolic
Acidosis
Expected pCO2 = 1.5 x [HCO3-]
+ 8 ± 2
Alkalosis
Expected pCO2 = 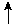 6
mmHg per 10 mEq/L
6
mmHg per 10 mEq/L 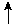 in HCO3-
in HCO3-
Respiratory
Acidosis
| Acute |
Expected 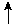 HCO3-
= HCO3-
= |
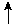 1
mEq/L for each 10 mm 1
mEq/L for each 10 mm 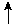 pCO2 pCO2 |
| Chronic |
Expected 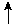 HCO3-
= HCO3-
= |
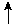 3.5mEq/L
for each 10 mmHg 3.5mEq/L
for each 10 mmHg 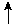 pCO2 pCO2 |
Alkalosis
| Acute |
Expected  HCO3-
= HCO3-
= |
 2
mEq/L for each 10 mm Hg 2
mEq/L for each 10 mm Hg  pCO2 pCO2 |
| Chronic |
Expected  HCO3-
= HCO3-
= |
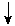 5
mEq/L for each 10 mmHg 5
mEq/L for each 10 mmHg 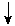 pCO2 pCO2 |
Some examples:
1. If the bicarbonate is 10 due to a purely metabolic acidosis, it
would be expected that the pCO2 would be about 23. If,
however, it were measured as 30, there must a component of respiratory
acidosis complicating the matter.
2. pH=7.08, pCO2=14, HCO3-=4,
Na=140, Cl=104:
-
Primary disorder is a metabolic acidosis
-
The pH is low indicating the primary disorder is acidosis.
-
The pCO2 is low, the expected compensation, trying to "blow
off" CO2.
-
The compensation does not fully correct the primary problem.
-
The predicted pCO2 by the above equation is 1.5*4+8 = 14.
-
This is the observed pCO2
-
The anion gap is 140 - (104 + 4) = 32, thus elevated.
Therefore, this is a simple increased anion gap metabolic acidosis.
3. pH 7.08, pCO2=14, HCO3-=4,
Na=140, Cl=124:
-
AG = 140 - (124 + 4) = 12
-
Same situation as above, but chloride has replaced bicarbonate.
4. pH 7.37, pCO2=18, HCO3-=10, Na=140,
Cl=114
-
For the pH to be normal, this must be mixed disorder (respiratory compensation
can never fully correct a simple metabolic acidosis).
-
The anion gap is 16, thus increased.
-
Expected pCO2 is 1.5*10+8 = 23 (21 at minimum).
-
Thus, there must be an element of respiratory alkalosis too.
Therefore, this is a combination of increased anion gap metabolic acidosis
and a respiratory acidosis.
5. In a patient with severe BPD, cor pulmonale, and who is on diuretics,
the pH=7.42, pCO2 = 65, HCO3-=41, Na 143,
K 3.1, Cl 88:
This chronic condition can be approached from either the viewpoint of
a a respiratory acidosis, or a metabolic alkalosis -- it doesn't matter
which one you start with, the result is the same: this is a mixed condition.
To prove it:
A) Start with a metabolic alkalosis, the patient has too much
bicarbonate...
-
The expected compensation would be retention of CO2, 6 mmHg
for each 10 mEq/L HCO3-.
-
Given a HCO3- of 41, with normal of 24: 41-24 = 17
-
Therefore, pCO2 should be 1.7 * 6 + 40 = 50.2 mmHg
-
However, pCO2 is measured at 65
-
Thus, there is a respiratory acidosis (due to CO2 retention)
which complicates the metabolic alkalosis which has come about secondary
to diurectic use.
B) Start with respiratory acidosis, the patient is a CO2
retainer...
-
The expected renal compensation would be a 3.5 mEq/L increase for every
10 mmHg increase in pCO2.
-
Thus, 65-40=25
-
The HCO3- should be 2.5*3.5+24 = 32.75
-
However, it is measured as 41
-
Thus, there is a metabolic alkalosis (due to diuretic use) which complicates
the respiratory acidosis due to CO2 retention.
Method II. Estimating by pH and pCO2
This method relies on following observation which is consistently true
for uncompensated respiratory conditions:
The pH varies by 0.008 units for every 1 mmHg
change in pCO2.
In children
-
the normal pH ranges from 7.35 to 7.45
-
the normal pCO2 ranges from 35 to 45
For a given condition, if the pCO2 makes sense in light of the
pH, the condition is of uncompensated respiratory origin. Metabolic
compensation for a primary respiratory condition usually takes between
8 and 48 hours to occur.
This is a useful way to analyze the situation when all you have is a
blood gas, and the bicarbonate value is not directly measured as in the
above examples.
Examples:
1. Given the ABG of 7.5/29/94/25 (pH/pCO2/pO2/HCO3-)
-
Since it is alkalemia, start at the upper end of normal, 7.45
-
Add 0.008 for each mmHg that the pCO2 differs from normal
-
The lower limit of normal pCO2 is 35, so
-
(35 - 29) = 6 mmHg less than normal pCO2
-
6 * 0.008 = 0.048
-
thus, predicted pH is 7.45 + 0.048 = 7.498
-
this jibes well with the measured 7.5
-
thus, this is an uncompensated respiratory alkalosis
2. Given the ABG of 7.2/64/75/25
-
Since it is acidemia, start at the lower limit of normal, 7.35
-
Subtract 0.008 for each mmHg that the pCO2 differs from normal
-
The upper limit of normal pCO2 is 45, so
-
(64-45) = 19 mmHg more than normal pCO2
-
19 * 0.008 = 0.152
-
thus, predicted pH is 7.35 - 0.152 = 7.198
-
this agrees well with the measured 7.2
-
thus, this is an uncompensated respiratory acidosis
3. Given the ABG of 7.31/72/52/35
-
Start with 7.35 because it is acidemia
-
The pCO2 differs from the maximum by (72 - 45) = 27
-
Expected pH = 7.35 - (0.008 * 27) = 7.13
-
Thus, this is not a simple respiratory acidosis; some compensation is present
4. Given the ABG of 7.50/59/60/41
-
The pH is elevated but the pCO2 is also elevated. This
cannot be a primary respiratory problem, but must be a metabolic alkalosis.
The degree to which each contribute requires additional information, either
a serum bicarbonate and the application of method
one (above) or interpretation of the base deficit.
Method III. Acid-Base Nomograms
The following nomogram can be used to classify a condition based
on blood gas measurements:

Adapted from: Goldberg, M., Green, S.B., Moss, M.L., et al. 1973.
JAMA223:269.
Copyright 1973, American Medical Association. |
Please direct all comments to: 
Last modification: April 30, 1998
6 mmHg per 10 mEq/L
in HCO3-
HCO3- =
1 mEq/L for each 10 mm
pCO2
HCO3- =
3.5mEq/L for each 10 mmHg
pCO2
HCO3- =
2 mEq/L for each 10 mm Hg
pCO2
HCO3- =
5 mEq/L for each 10 mmHg
pCO2
